SPECIFICATION OF GENETIC-BASED SYSTEMIC MANIFESTATIONS
OF HUMAN TENDENCY TO AGGRESSIVE, SUICIDAL AND ADDICTION BEHAVIOR
JOURNAL: «SCIENTIFIC NOTES OF V.I. VERNADSKY CRIMEAN FEDERAL UNIVERSITY. Biology. Chemistry» Volume 10 (76), №2, 2024
Publication text (PDF):Download
UDK: 575.16+316.624
AUTHOR AND PUBLICATION INFORMATION
AUTHORS:
Mulik A. B., Military Medical Academy, St. Petersburg, Russia
Shatyr Yu. A., Military Medical Academy, St. Petersburg, Russia
Nazarov N. O., Center for Implementation of Changes of the Ministry of Health of the Moscow Region, Krasnogorsk, Moscow region, Russia
Trandina A. E., Military Medical Academy, St. Petersburg, Russia
Buntovskaya A. S., Military Medical Academy, St. Petersburg, Russia
Ulesikova I. V., Military Medical Academy, St. Petersburg, Russia
Glushakov R. I. Military Medical Academy, St. Petersburg, Russia
TYPE:Article
DOI:https://doi.org/10.29039/2413-1725-2024-10-2-128-143
PAGES:from 128 to 143
STATUS:Published
LANGUAGE:English
KEYWORDS: SNP, genotypes, genetics of aggressive behavior, genetics of suicidal behavior, genetics of addictive behavior.
ABSTRACT (ENGLISH):
INTRODUCTION
As is known, any forms of complex, including deviant, behavior are formed under the influence of a complex of biological, psychological, social, natural and man-made factors. Moreover, the entire set of endogenous factors is certainly determined by many genes that interact systematically and ensure stable manifestations of a person’s phenotypic characteristics, potentially predetermining his tendency to certain forms of deviant behavior [1]. Individual sets of genetic and phenotypic characteristics demonstrate the stability of the propensity for certain deviations in a particular person throughout his life, despite the fact that the peak of behavioral deviations occurs in adolescence [2].
The current state of research into the genetic determination of individual sets of behavioral deviations confirms the advisability of further searching for the genetic foundations of the systemic formation of various vectors of deviant behavior. A number of works present systemic connections between sets of genetic and phenotypic factors in the complex manifestation of behavioral deviations. Thus, the studies of E.A.D. Clifton et al. (2018) and J. Tiebeek et al. (2022) substantiate the systematic genetic determination of indicators of social behavior, mental health, physical well-being, addictiveness, cognition, level of education, reproductive characteristics [3, 4]. L. R. Karlsson et al. (2019) summarized evidence of shared genetic influences on measures of risk tolerance and risky behavior [5]. M. A. Spano et al. (2023) found a negative correlation between genetically determined risky behavior (smoking, drinking alcohol, lack of physical activity) and the desire for education [6].
Previously conducted own studies revealed stable combinations of phenotypic indicators (high level of general nonspecific reactivity of the organism, excitability, anxiety, depression, adventurousness, affectiveness, neuroticism, irritability), which are complexly correlated with aggressive, suicidal and addictive behavior. These behavioral deviations are the basis of social and criminal tension in society, which justifies the need for further study of their etiology.
As a result of summarizing our own research [7–11] and the latest data from the scientific literature [12–16], the following candidate genes and corresponding polymorphisms were identified, systemically associated with phenotypic signs of a person’s tendency to aggression, auto-aggression and chemical addictions: DRD2 (rs1800497), DRD3 (rs6280), CACNA2D3-1 (rs1851048), CACNA2D3-2 (rs6777055), COMT (rs4680), ZNF-LD (rs2562456), GRM1 (rs6923492).
The purpose of our study was to characterize the genetic determination of the systemic manifestation of a person’s tendency to aggressive, suicidal and addictive behavior.
MATERIALS AND METHODS
The study involved 300 clinically healthy men and women 18–25 years old, representatives of the Caucasian race, indigenous residents of three regions of the European part of Russia: Arkhangelsk region, Volgograd region, Republic of Crimea. For the study, we selected students from state universities who were brought up in a complete, socially prosperous family, without financial and everyday problems, and without chronic somatic and neurological diseases. Все работы проводили анонимно, в апреле-мае 2023 года. All studies were conducted anonymously in April-May 2023. The principles of the Universal Declaration of Bioethics and Human Rights (Articles 4 (benefit and harm), 5 (autonomy and individual responsibility), 6 (consent) and 9 (privacy and confidentiality) were observed [4].
The psychological status of the study participants was determined by assessing the Freiburg Multifactor Personality Inventory – FPI (I. Farenberg, H. Zarg, R. Gampel) [17]; character accentuations (K. Leonhard [18]); suggestibility, frustration, irritability and resentment (V. V. Kozlov et al. [19]); adventurousness (A. Chichin [20]); behavioral, social, professional, economic, political activity and social destructiveness (Yu.A. Shatyr et al. [21]); type of behavioral activity (cardiotype) A–B (V. V. Delarue and F. A. Tambieva [22]). The tendency to auto-aggression was determined according to the method of T.N. Razuvaeva, the severity of suicidal ideation was assessed using the suicidal ideation module of the Columbia Suicidal Severity Scale (C-SSRS) [23]. To assess the behavioral status associated with chemical addictions, the experience and frequency of alcohol consumption, smoking and drug use were identified through a survey.
Laboratory genetic research of biological material was carried out by real-time polymerase chain reaction (PCR) method using kits produced by Synthol (Russia) and a real-time amplifier RotorGene 6000 (Corbett Research, Australia). Genomic DNA was isolated from buccal epithelium by adsorption onto magnetic particles. The following polymorphisms were studied that are promising in relation to aggression, autoaggression and chemical addictions: rs1800497 (DRD2), rs6280 (DRD3), rs1851048 (CACNA2D3-1), rs6777055 (CACNA2D3-2), rs4680 (COMT), rs2562456 (ZNF-LD), rs6923492 (GRM1).
To perform statistical analysis, the pandas, matplotlib.pyplot, phik, numpy, seaborn, scipy packages of the Python programming language were used. Correlation analysis was performed using the Phi K Correlation and Global Correlations method with the calculation of coefficients фk and gk. Comparison of polymorphisms was performed using a nonparametric method – the Kruskal-Wallis test.
RESULTS AND DISCUSSION
To identify the main, most pronounced systemic connections between indicators of the risk of developing aggression, auto-aggression and chemical addictions with the analyzed polymorphisms, global correlation coefficients were calculated for the entire sample population of subjects. Only indicators that had a statistically significant relationship with at least one polymorphism under study were taken into account (Figure 1).
The next stage of the study was devoted to a comparative analysis of the severity of risk indicators for the development of aggression, auto-aggression and chemical addictions between groups of subjects with different genotypes for each polymorphism studied. Indicators that showed statistically significant differences or trends towards statistically significant differences (p<0.1) between the genotypes of the analyzed SNP were selected for the final accounting. The results of the study are presented in Figures 2, 3, 4, 5, 6, 7, 8.
It is known from the literature that the minor allele of the rs1800497 polymorphism (T allele) is associated with a reduced number of dopamine binding sites in the brain and presumably determines the presence of alcohol and nicotine addiction, as well as predisposition to a number of neuropsychiatric disorders, including eating disorders [24–26]. The highest risk of alcohol dependence was identified in the C/T genotype, along with the risk of obesity and suicidal tendencies [27]; the T/T genotype has a higher risk of developing attention deficit hyperactivity disorder, less pronounced pleasure reactions, and a higher likelihood of developing depression, while the C/C genotype, along with a high probability of developing alcohol dependence, has a risk of developing attention deficit hyperactivity disorder, along with a high level of emotional intelligence [14, 16].
The presented data from the experimental study (Figure 2) confirmed the connection of the T allele with nicotine addiction, but the connection of the C/C genotype with alcoholism was not determined. At the same time, in the C/C genotype variant (“major” or the most common in the European race) was revealed the minimal severity of systemic manifestations of indicators of aggressiveness, antisocial behavior and tobacco consumption in relation to the S/T variant.
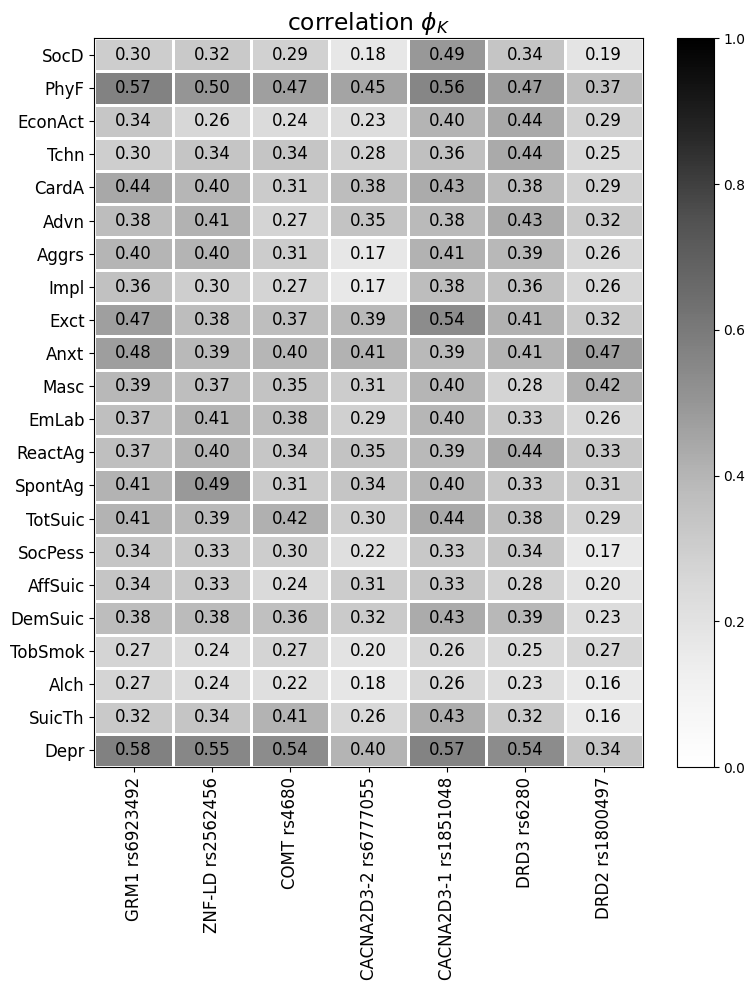
Rice. 1. Correlation coefficients Phi K of the analyzed polymorphisms with risk indicators of developing aggression, auto-aggression and chemical addictions
A.M.F. Pego et al. (2020) found an association between the T/C and C/C rs6280 genotypes with drug addiction, as well as with a predisposition to risky behavior, including aggressive behavior [28]. C. Zhao et al. (2016) revealed a relationship between rs6280 and social conformity: it was found that in individuals with the genotype variant of the C/C polymorphism, which is characterized by increased release of dopamine in the striatum, susceptibility to social influence is more pronounced relative to individuals with genotypes C/T and T/T [29].
Fig. 2. The severity of indicators of a person’s propensity to aggression, suicide and chemical addictions in various genotypes SNP rs1800497 (DRD2)
Note: T/T genotype is not included in the sample; * – p ≤0,1;** – p≤0,05; *** – p≤0,001
The results of the experimental study (Figure 3) confirm the influence of the T/C and C/C genotypes on a person’s tendency to aggressive behavior. Susceptibility to the social environment among representatives of the C/C genotype is high, the level of their demonstrative suicidality against the background of a low level of suicidal ideas. At the same time, in the variant of the T/T genotype (“major” or the most common in the European race) the minimum severity of the systemic manifestation of indicators of aggressiveness, suicidality, irritability and resentment is determined.
SNP rs1851048 of the CACNAD3-1 gene is the least studied in relation to the systemic connections of its genotypes with aggressive, suicidal and addictive behavior. Single studies in this direction demonstrate an indirect connection of rs1851048 with risky behavior [30–32].
The results of the undertaken experimental study (Figure 4) first demonstrated systematic relationships between risk indicators for the development of aggression, auto-aggression and chemical addictions with rs1851048 genotypes. In general, the positive influence of the major G/G genotype on the entire range of behavioral, functional and psychological grounds for the minimal risk of aggressive, suicidal and addictive actions in humans is confirmed.
Rice. 3. The severity of indicators of a person’s propensity to aggression, suicide and chemical addictions in different genotypes SNP rs6280 (DRD3)
Note: * – p ≤0,1;** – p≤0,05; *** – p≤0,001
Based on the literature, SNP rs 6777055 CACNA2D3-2 is associated with depression, neuroticism, and emotional lability [33]. Однако, информация в отношении системных связей его генотипов с агрессивным, суицидальным и аддиктивным поведением в доступных источниках отсутствует. However, information regarding the systemic connections of its genotypes with aggressive, suicidal and addictive behavior is not available in available sources. Overall, CACNAD3-1 and CACNA2D3-2 are considered to be genes associated with a wide variety of neurological and neuropsychiatric disorders, including depressive disorders [34].
Fig. 4. The severity of indicators of a person’s propensity for aggression, suicide and chemical addictions in various genotypes SNP rs1851048 (CACNA2D3-1)
Note: * – p ≤0,1;** – p≤0,05; *** – p≤0,001
The presented results of the experimental study (Figure 5) to a greater extent demonstrate the connection of the minor genotype C/C rs 6777055 with a pronounced tendency to aggressive behavior, against the background of a minimal risk of suicidality and manifestations of aggressiveness in representatives of the A/A genotype.
Fig. 5. The severity of indicators of a person’s propensity to aggression, suicide and chemical addictions in various genotypes SNP rs6777055 (CACNA2D3-2)
Note: * – p ≤0,1;** – p≤0,05
M. Kaminskaite et al. (2020) found an association of rs4680 with the risk of alcohol dependence [35]. The G (Val) allele increases the risk of depression. Individuals with the G/G genotype are found to have a predisposition to risky behavior, higher levels of depression, a tendency to obesity and type 2 diabetes, as well as to consume foods rich in fat. For the G/A genotype (the most common heterozygous polymorphism in the population), an average level of predisposition to risky behavior was noted, and for the A/A genotype, more persistent associations with bulimia nervosa, anxiety, risk avoidance, as well as a higher level of emotional intelligence were noted [36–38].
The results of the experimental study (Figure 6) confirm the influence of the G/G genotype on a person’s tendency to engage in risky behavior, due to a high level of social destructiveness, irritability, aggressiveness and suicidality. The role of the A/A genotype in the formation of anxiety and minimization of risk behavior, including those caused by low levels of psychoactive substance consumption, has been confirmed.
As follows from the literature data, among the possible rs2562456 genotypes, the A/A genotype in men has a statistically significant connection with such indicators of psychological status as balance and masculinity. In women with the G/G genotype, a statistically significant relationship with the pain sensitivity threshold was revealed [32, 39]. This indirectly indicates the positive impact of these genotypes (A/A and G/G) on a person’s psycho-emotional state and minimization of risky behavior factors.
The presented results of the experimental study (Figure 7) do not confirm the positive relationship of the A/A genotype with masculinity in the general sample of men and women. For the first time, in relation to representatives of the A/G genotype, the maximum severity of irritability, impulsivity, as well as affective, demonstrative and general suicidality was revealed. Against this background, the minimum values of the systemic manifestation of indicators of aggressiveness and suicidality are characterized by persons with the A/A genotype.
Fig. 6. Expression of indicators of human propensity to aggression, suicide and chemical addiction in various genotypes of SNP rs4680 (COMT)
Note: * – p ≤0,1;** – p≤0,05
Fig. 7. The severity of indicators of a person’s propensity to aggression, suicide and chemical addictions in various genotypes SNP rs2562456 (ZNF-LD)
Note: * – p ≤0,1;** – p≤0,05; *** – p≤0,001
There is limited data on SNP rs6923492 of the GRM1 gene that substantiates its association with prenatal risk factors for the development of attention deficit disorder and autism spectrum disorders [40, 41]. Its influence on the development of externalizing behavior (challenging behavior), on learning disorders, and in adult life on mood disorders, the development of anxiety, and the demand for psychoactive substances has been shown [15, 42, 43].
Fig. 8. The severity of indicators of a person’s propensity for aggression, suicide and chemical addictions in various genotypes SNP rs6923492 (GRM1)
Note: * – p ≤0,1;** – p≤0,05; *** – p≤0,001
The presented data from the experimental study (Figure 8) for the first time shows the systematic relationships between risk indicators for the development of aggression, auto-aggression and chemical addictions with rs923492 genotypes. In general, the positive influence of the heterozygous T/S genotype on the entire range of behavioral, functional and psychological grounds for the minimal risk of aggressive, suicidal and addictive actions in humans has been proven.
Based on the results of the analysis of the potential role of the identified single nucleotide polymorphisms in the formation of the phenotypic prerequisites for a person’s systemic tendency to aggressive, suicidal and addictive behavior, the genotypes of the presented SNPs were characterized by the presence and direction of their influence on the studied vectors of deviant behavior (Table).
Table
Genotypic characteristics of the systemic manifestation of a person’s tendency to aggressive, suicidal and addictive behavior
| SNP (gene) | Genotype | Deviations | ||
| Aggressiveness | Suicidalness | Addictiveness | ||
| 1800497 (DRD2) | C/T | (+) | (0) | (+) |
| T/T | no data | no data | no data | |
| C/C | (-) | (0) | (-) | |
| 6280
(DRD3) |
T/C | (0) | (0) | (0) |
| C/C | (+) | (+) | (0) | |
| T/T | (-) | (-) | (0) | |
| 1851048 (CACNAD3-1) | A/A | (0) | (+) | (0) |
| G/G | (-) | (-) | (-) | |
| G/A | (+) | (0) | (+) | |
| 6777055 (CACNA2D3-2) | A/A | (-) | (-) | (0) |
| C//C | (+) | (0) | (0) | |
| A/C | (0) | (+) | (0) | |
| 4680
(COMT) |
G/A | (+) | (0) | (+) |
| G/G | (0) | (+) | (+) | |
| A/A | (-) | (-) | (-) | |
| 2562456
(ZNF-LD) |
A/A | (-) | (-) | (0) |
| G/G | (+) | (0) | (0) | |
| A/G | (0) | (+) | (0) | |
| 6923492
(GRM1) |
С/С | (+) | (+) | (+) |
| Т/С | (-) | (-) | (-) | |
| Т/Т | (+) | (+) | (+) | |
Note: (+) – positive connection; (-) – negative connection; (0) – no connection.
CONCLUSIONS
As a result of the undertaken research, systemic connections between risk indicators of aggressive, suicidal and addictive behavior with polymorphisms and corresponding human genotypes were determined. An information table has been developed that specifically reflects the relationship between the genotypes of the identified polymorphisms and the presence and direction of their influence on the studied vectors of deviant behavior.
The work was carried out as part of the project “Forecasting the risks of developing aggressive, suicidal and addictive behavior among the population of territories with different physical-geographical and biogeochemical status” under the academic strategic leadership program “Priority–2030”.
- Jansen L. M. C. The neurobiology of antisocial behavior in adolescence; current knowledge and relevance for youth forensic clinical practice. Curr Opin Psychol, 47, 101356 (2022).
- Josef A. K., Richter D., Samanez-Larkin G. R., Wagner G. G., Hertwig R. and Mata R. Stability and change in risk-taking propensity across the adult life span. J Pers Soc Psychol, 111(3), 430 (2016).
- Clifton E. A. D., Perry J. R. B., Imamura F., Lotta L. A., Brage S., Forouhi N. G., Griffin S. J., Wareham N. J., Ong K. K. and Day F. R. Genome–wide association study for risk taking propensity indicates shared pathways with body mass index. Communications Biology, 3(1), 36 (2018).
- Tielbeek J. J., Uffelmann E., Williams B. S., Colodro-Conde L., Gagnon É., Mallard T. T., Levitt B. E., Jansen P. R., Johansson A., Sallis H. M., Pistis G., Saunders G. R. B., Allegrini A. G., Rimfeld K., Konte B., Klein M., Hartmann A. M., Salvatore J. E., Nolte I. M., Demontis D., Malmberg A. L. K., Burt S. A., Savage J. E., Sugden K., Poulton R., Harris K. M., Vrieze S., McGue M., Iacono W. G., Mota N. R., Mill J., Viana J. F., Mitchell B. L., Morosoli J. J., Andlauer T. F. M., Ouellet-Morin I., Tremblay R. E., Côté S. M., Gouin J. P., Brendgen M. R., Dionne G., Vitaro F., Lupton M. K., Martin N. G.; COGA Consortium; Spit for Science Working Group; Castelao E., Räikkönen K., Eriksson J. G., Lahti J., Hartman C. A., Oldehinkel A. J., Snieder H., Liu H., Preisig M., Whipp A., Vuoksimaa E., Lu Y., Jern P., Rujescu D., Giegling I., Palviainen T., Kaprio J., Harden K. P., Munafò M. R., Morneau-Vaillancourt G., Plomin R., Viding E., Boutwell B. B., Aliev F., Dick D. M., Popma A., Faraone S. V., Børglum A. D., Medland S. E., Franke B., Boivin M., Pingault J. B., Glennon J. C., Barnes J. C., Fisher S. E., Moffitt T. E., Caspi A., Polderman T. J. C. and Posthuma D. Uncovering the genetic architecture of broad antisocial behavior through a genome-wide association study meta-analysis. Mol Psychiatry, 27(11), 4453 (2022).
- Karlsson L. R., Biroli P., Kong E., Meddens S. F. W., Wedow R., Fontana M. A., Lebreton M., Tino S. P., Abdellaoui A., Hammerschlag A. R., Nivard M. G., Okbay A., Rietveld C. A., Timshel P. N., Trzaskowski M., Vlaming R., Zünd C. L., Bao Y., Buzdugan L., Caplin A. H., Chen C. Y., Eibich P., Fontanillas P., Gonzalez J. R., Joshi P. K., Karhunen V., Kleinman A., Levin R. Z., Lill C. M., Meddens G. A., Muntané G., Sanchez-Roige S., Rooij F. J. V., Taskesen E., Wu Y., Zhang F.; 23and Me Research Team; eQTLgen Consortium; International Cannabis Consortium; Social Science Genetic Association Consortium; Auton A., Boardman J. D., Clark D. W., Conlin A., Dolan C. C., Fischbacher U., Groenen P. J. F., Harris K. M., Hasler G., Hofman A., Ikram M. A., Jain S., Karlsson R., Kessler R. C., Kooyman M., MacKillop J., Männikkö M., Morcillo-Suarez C., McQueen M. B., Schmidt K. M., Smart M. C., Sutter M., Thurik A. R., Uitterlinden A. G., White J., Wit H., Yang J., Bertram L., Boomsma D. I., Esko T., Fehr E., Hinds D. A., Johannesson M., Kumari M., Laibson D., Magnusson P. K. E., Meyer M. N., Navarro A., Palmer A. A., Pers T. H., Posthuma D., Schunk D., Stein M. B., Svento R., Tiemeier H., Timmers P. R. H. J., Turley P., Ursano R. J., Wagner G. G., Wilson J. F., Gratten J., Lee J. J., Cesarini D., Benjamin D. J., Koellinger P. D. and Beauchamp J. P. Genome-wide association analyses of risk tolerance and risky behaviors in over 1 million individuals identify hundreds of loci and shared genetic influences. Nat Genet, 51(2), 245 (2019).
- Spano M. A., Morris T., Davies N. M. and Hughes A. Genetic association of risk behaviours and educational attainment. Research Square. Under review (2023). https://doi.org/10.21203/rs.3.rs-2851416/v1
- Shatyr Yu. A., Bondarev A. M., Novochadov V. V. and Mulik A. B. Virtual Screening SNP-Polymorphisms of Genes Determining the High Level of General Non-Specific Reactivity of Organism. European Journal of Molecular Biotechnology. 3(9), 174 (2015).
- Mulik A. B., Shatyr Yu. A., Bondarev A. M. and Nazarov N. O. Analysis of individual aspects of phenotypic and genotypic determination of impulsive human behavior. Medline.ru. Russian biomedical journal, 16(3), 445 (2015) (In Russ.).
- Mulik A. B., Shatyr Yu. A., Ulesikova I. V. and Nazarov N. O. Systemic mechanisms of population formation of a person’s propensity to consume alcohol and smoke tobacco: monograph. 184 p. (Pero Publishing House, Moscow, 2022) (In Russ.).
- Krylov P. A., Gerasimova E. O., Shatyr Yu. A. and Mulik A. B. Virtual screening SNP of genes associated with the risk of using psychoactive substances taking into account different phenotype signs. Scientific Notes of V.I. Vernadsky Crimean Federal University. Biology. Chemistry, 7(4), 69 (2021) (In Russ.).
- Mulik A. B. Systemic mechanisms of population formation of human behavioral and social activity: monograph. 152 p. (RUSAINS, Moscow, 2017) (In Russ.).
- Blum K., Bowirrat A., Elman I., Baron D., Thanos P. K., Gold M. S., Hanna C., Makale M. T., Sunder K., Jafari N., Zeine F., Murphy K. T., Makale M. and Badgaiyan R. D. Evidence for the DRD2 Gene as a Determinant of Reward Deficiency Syndrome (RDS). Clin Exp Psychol, 29(9(4)), 8 (2023).
- Zhang X., Han Y., Liu X., Chen J., Yuan Z. and Wang Y. Assessment of genetic variants in D2 dopamine receptor (DRD2) gene as risk factors for post-traumatic stress disorder (PTSD) and major depressive disorder (MDD): A systematic review and meta-analysis. J Affect Disord, 328, 312 (2023).
- Daza-Hernández S., Pérez-Luque E., Martínez-Cordero C., Figueroa-Vega N., Cardona-Alvarado M. I. and Muñoz-Montes N. Analysis of Factors Associated with Outcomes of Bariatric Surgery: rs1800497 ANKK1, rs1799732 DRD2 Genetic Polymorphisms, Eating Behavior, Hedonic Hunger, and Depressive Symptoms. J Gastrointest Surg, 27(9), 1778 (2023).
- da Silva B. S., Grevet E. H., Silva L. C. F., Ramos J. K. N., Rovaris D. L. and Bau C. H. D. An overview on neurobiology and therapeutics of attention-deficit/hyperactivity disorder. Discover Mental Health, 3(1), 2 (2023).
- Gafarov V. V., Gromova E. A., Panov D. O., Maximov V. N., Gagulin I. B. and Gafarova A. V. Association of the polymorphic marker Val158Met of the COMT gene with depression in an open population 25-44 years old (WHO international program MONICA, epidemiological study). Neurology, neuropsychiatry, psychosomatics, 13(2), 19 (2021) (In Russ.).
- http://psylab.info/ [Internet]. Frajburgskij lichnostnyj oprosnik. Available from: http://psylab.info/ Freiburg Personality Questionnaire/Test Material (Form B) (In Russ.).
- Leongard K. Accented personalities. 446 p. (EXMOPress, Moscow, 2001) (In Russ.).
- Kozlov V. V., Mazilov V. A. and Fetiskin N. P. Socio-psychological diagnostics of personality development and small groups. 2nd edition, expanded and revised. 720 p. (Institute of Psychotherapy and Clinical Psychology, Moscow, 2018) (In Russ.).
- Chichin A. Test for adventurism. Character development. Access via link:http://harakter.into/index.php/lyudov/19-testy-poharakteru/test-urovenngativnykh-chert-kharaktera/85-test-naavantyurizm (In Russ.).
- Shatyr Yu. A., Mulik I. G., Ulesikova I. V., Bulatetskiy and Mulik A. B. Optimization of assessing the severity and direction of human social activity. Science of the Young (Eruditio Juvenium), 5(4), 393 (2017). (In Russ.).
- Delarue V. V. and Tambieva F. A. Methods for studying personality. 114 p. (Rostov State University Publishing House, Kislovodsk, 1998) (In Russ.).
- Soldatkin V. A., Perekhov A. Ya., Trufanova O. K., Bukhanovskaya O. A., Vychuzhina Y. V., Zagoruiko E. N., Zotov P. B., Dyakova I. V., Kashin A. A. A., Kovalev A. I., Kryuchkova M. N., Letifova N. G., Mavani D. Ch., Malyshko E. V., Mrykhin V. V. and Tarakanova E. A. Clinical psychometrics. 352 p. (Rostov State Medical University Publishing House, Rostov-on-Don, 2020) (In Russ.).
- Spitta G., Fliedner L. E., Gleich T., Zindler T., Sebold M., Buchert R., Heinz A., Gallinat J. and Friedel E. Association between DRD2/ANKK1 TaqIA Allele Status and Striatal Dopamine D2/3 Receptor Availability in Alcohol Use Disorder. J Integr Neurosci, 21(6), 171 (2022).
- Aliasghari F., Mahdavi R., Barati M., Nazm S. A., Yasari S., Bonyadi M., Jabbari M. Genotypes of ANKK1 and DRD2 genes and risk of metabolic syndrome and its components: A cross-sectional study on Iranian women. Obes Res Clin Pract, 15(5), 449 (2021).
- Aliasghari F., Nazm S. A., Yasari S., Mahdavi R. and Bonyadi M. Associations of the ANKK1 and DRD2 gene polymorphisms with overweight, obesity and hedonic hunger among women from the Northwest of Iran. Eat Weight Disord, 26(1), 305 (2021).
- Hidalgo Vira N., Oyarce K., Valladares Vega M., Goldfield G. S., Guzmán-Gutiérrez E. and Obregón A. M. No association of the dopamine D2 receptor genetic bilocus score (rs1800497/rs1799732) on food addiction and food reinforcement in Chilean adults. N. Front Behav Neurosci, 17, 1067384 (2023).
- Pego A. M. F., Leyton V., Miziara I. D., Bortolin R. H., Freitas R. C. C., Hirata M., Tomaz P. R. X., Santos J. R., Santos P. C. J. L. and Yonamine M. SNPs from BCHE and DRD3 genes associated to cocaine abuse amongst violent individuals from Sao Paulo, Brazil. Forensic Sci Int, 317, 110511 (2020).
- Zhao C., Liu J., Gong P., Hu J. and Zhou X. Investigating the Genetic Basis of Social Conformity: The Role of the Dopamine Receptor 3 (DRD3) Gene. Neuropsychobiology, 74(1), 32 (2016).
- Rhodin A., Grönbladh A., Ginya H., Nilsson K. W., Rosenblad A., Zhou Q., Enlund M., Hallberg M., Gordh T. and Nyberg F. Combined analysis of circulating β-endorphin with gene polymorphisms in OPRM1, CACNAD2 and ABCB1 reveals correlation with pain, opioid sensitivity and opioid-related side effects. Mol Brain, 6, 8 (2013).
- Shatyr Yu. A., Nazarov N. O., Glushakov R. I., Ulesikova I. V., Kukhtalev V. V. and Mulik A. B. Search for genetic and phenotypical bases of human predisposition to risk behavior. Scientific Notes of Crimean V. I. Vernadsky Federal University Biology. Chemistry, 9(75(3)), 291 (2023).
- Mulik A., Novochadov V., Bondarev A., Lipnitskaya S., Ulesikova I. and Shatyr Yu. New insights into genotype-phenotype correlation in individuals with different level of general non-specific reactivity of an organism. Journal of Integrative Bioinformatics, 13(4), 295 (2016).
- Spasova A. P., Barysheva O. Yu. and Tikhova G. P. Polymorphism of the catechol-o-methyltransferase gene and pain. Regional anesthesia and treatment of acute pain. 11(1), 6 (2017).
- Platonkina T. V., Bogovin L. V., Naumov D. E. and Ovsyankin A. I. Genetic studies of depressive disorders: review of the literature. Bulletin of Physiology and Pathology of Respiration, 68, 96 (2018) (In Russ.).
- Kaminskaite M., Jokubka R., Janaviciute J., Lelyte I., Sinkariova L., Pranckeviciene A., Borutaite V. and Bunevicius A. Epistatic effect of Ankyrin repeat and kinase domain containing 1 — Dopamine receptor D2 and catechol-o-methyltransferase single nucleotide polymorphisms on the risk for hazardous use of alcohol in Lithuanian population. Gene, 765, 145107 (2021).
- Motalova Yu. I. and Vorobyova E. V. The role of genes of the serotonergic and dopaminergic systems in the occurrence of eating disorders: a review of modern research. Innovative science: Psychology, Pedagogy, Defectology, 1(2), 133 (2018) (In Russ.).
- Vasilyeva A. A., Vasiliev V. A., Okushko R. V. and Negasheva M. A. Associations of polymorphism of the catechol-O-methyltransferase (COMT) gene with morphofunctional indicators in students of Russia and Transnistria». Molecular Genetics, Microbiology and Virology, 1, 42 (2021) (In Russ.).
- Vorobyova E. V., Kovsh E. M. and Kosonogov V. V. Emotional intelligence in carriers of different genotypes of COMT, BDNF, DRD2 AND HTR2A. Psychological analysis, 15(2), 83 (2022) (In Russ.).
- Mulik A. B., Yusupov V. V., Nazarov N. O., Ulesikova I. V., Sroslova G. A. and Shatyr Yu. A. Conditions for the formation of motivation for alcohol and tobacco consumption. Preventive Medicine, 26(2), 106 (2023) (In Russ.).
- Waltes R., Freitag C. M., Herlt T., Lempp T., Seitz C., Palmason H., Meyer J. and Chiocchetti A. G. Impact of autism-associated genetic variants in interaction with environmental factors on ADHD comorbidities: an exploratory pilot study. J Neural Transm (Vienna), 126(12), 1679 (2019).
- Waltes R., Duketis E., Knapp M., Anney R. J., Huguet G., Schlitt S., Jarczok T. A., Sachse M., Kämpfer L. M., Kleinböck T., Poustka F., Bölte S., Schmötzer G., Voran A., Huy E., Meyer J., Bourgeron T., Klauck S. M., Freitag C. M. and Chiocchetti A. G. Common variants in genes of the postsynaptic FMRP signalling pathway are risk factors for autism spectrum disorders. Hum Genet, 133(6), 781 (2014).
- Akmatov M. K., Ermakova T. and Bätzing J. Psychiatric and nonpsychiatric comorbidities among children with ADHD: an exploratory analysis of nationwide claims data in Germany. J Atten Disord, 25, 874 (2021).
- Chen Q., Hartman C. A., Haavik J., Harro J., Klungsøyr K., Hegvik T. A., Wanders R., Ottosen C., Dalsgaard S., Faraone S. V. and Larsson H. Common psychiatric and metabolic comorbidity of adult attention-deficit/hyperactivity disorder: a population-based cross-sectional study. PLoS ONE, 13(9), e0204516 (2018).

